Our Labs & Infrastructure for ATMP Development
CCRM Nordic was established to address the critical infrastructure gap for ATMP development and manufacturing in the Nordics. We are bridging this gap with our operational development labs and our upcoming large-scale development and GMP manufacturing facility.
A Life Science Powerhouse with Global Reach
CCRM Nordic is one of several hubs in the CCRM network with the aim to coordinate and optimize opportunities in the field of advanced therapies.

Our Current Labs & Facilities
Located adjacent to our offices in GoCo House, our interim 200 m2 lab is equipped to handle process and analytical development projects. This facility allows us to de-risk and optimize manufacturing workflows in preparation for future GMP translation.
CAR-T Manufacturing Workflow
Our recent focus has been on establishing an industry gold-standard CAR-T manufacturing workflow, including assays for typical quality attributes.
Key Equipment and Capabilities
We operate state-of-the-art bioprocessing equipment to support a wide range of development needs. This includes systems such as the Sefia Select, the Xuri Cell Expansion System, the CliniMACS Prodigy, G-Rex Bioreactors, the Ambr 250 Modular, and many more.

Our Future Facility: An Integrated Hub for GMP and Development
Our new facility will house our offices, next-generation development labs, and a full-service GMP manufacturing area, including dedicated QC labs. We are already supporting projects that are moving towards clinical supply to be manufactured in this future facility.
-
Next-Generation Development Labs (~1000 m2)
In our new, larger development labs, we will build upon our existing capabilities. This will allow us to work on more projects concurrently, develop platforms for additional modalities, and operate at larger bioreactor scales.
-
GMP Manufacturing Facility (~1000 m2)
The GMP facility will allow us to provide an integrated manufacturing service offering, complemented by a comprehensive panel of analytical assays from our dedicated QC labs. Services will include GMP cell banking, GMP manufacturing, fill-finish, full QA and QP release, stability studies, and tech transfer to a late-stage partner.
-
Designed for Seamless and Efficient Tech Transfer
The close proximity of the development labs to the GMP manufacturing and QC areas is by design. This co-location ensures that tech transfer will be as simple, efficient, and robust as possible.
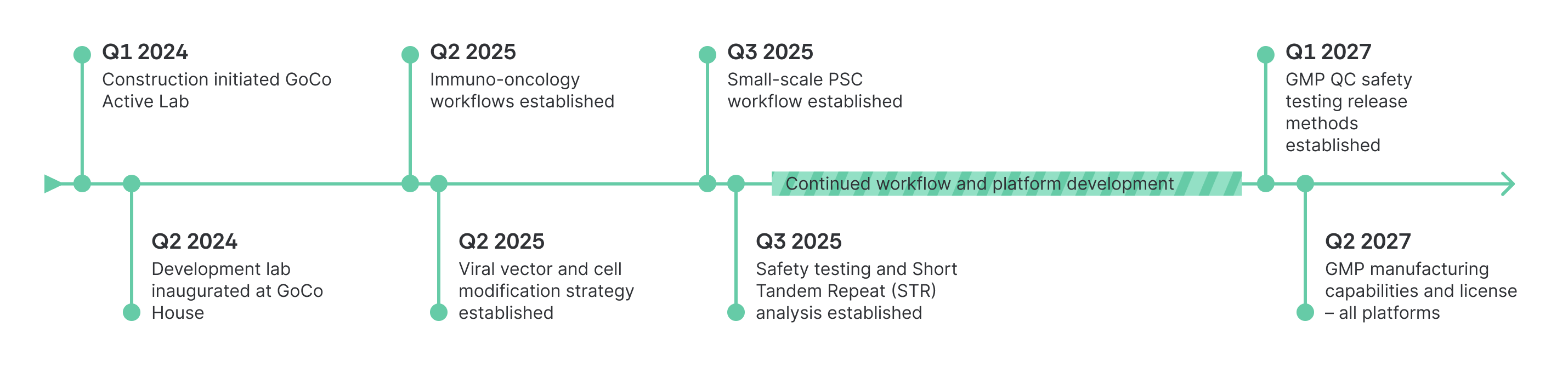
Timeline & Construction Milestones
Our new facility will house our offices, next-generation development labs, and a full-service GMP manufacturing area, including dedicated QC labs. Building on the development labs and workflows already established in our current facility, we are now supporting projects that are moving towards clinical supply to be manufactured in this future facility.
Stay Connected with CCRM Nordic
Join our network for exclusive insights, career opportunities, and thought leadership content from Nordic industry experts.
- 4000+ professionals
- Direct communication
- Weekly insights
