GMP Manufacturing
Our purpose-built ATMP production site will combine an innovative facility design with a team dedicated to the rigour and excellence required for GMP operations.
- Q2 2027: 1000 m2 GMP facility operational
- Expertise in viral and cell-based therapies
- Autologous and allogeneic supply chains
Let's discuss how we can suppport your path to GMP manufacturing!
Support for Your Clinical Journey
Our GMP facility is currently under construction and will be operational in the second half of 2027. We are actively planning manufacturing campaigns with clients for this timeline. Our development labs, however, are fully functional, enabling process and analytical development today that will be ready for seamless tech transfer to GMP. For immediate needs, we have an interim GMP manufacturing agreement with our partner, SCTbio.
We’ve set up our immune cell therapy workflows and generated some interesting data we’re happy to share with you in a poster. Reach out to us if you’d like to hear more about the cost of manufacturing comparisons we’ve done for the featured platforms.

Our Future Facilities Will Be Ready by Q2 2027
Our new 1000 m2 GMP site is being built within the GoCo Health Innovation City in Gothenburg. It will feature one grade B room, four grade C rooms, and will be co-located with our QC and development labs to enable seamless tech transfer. While our initial focus is on early-stage clinical supply, we have partners for a smooth transition to late-stage and commercial supply.
GMP Manufacturing
We will provide an integrated service offering for cell- and viral-based therapies, including GMP cell banking and fill-finish.
QA & QP Release
Our services will be complemented by a comprehensive panel of analytical assays performed in our QC labs, along with full QA and QP release.
Stability & Tech Transfer
We will manage stability studies and offer a clear pathway for tech transfer to a late-stage manufacturing partner when you are ready to scale.
The Critical Role of GMP in ATMP Development
Good Manufacturing Practice (GMP) is essential for the clinical application of ATMPs, ensuring products are consistently produced and controlled to the highest quality standards.
GMP compliance is a mandatory requirement to guarantee patient safety and to progress your therapy from research to clinical trials and, ultimately, to market authorisation.
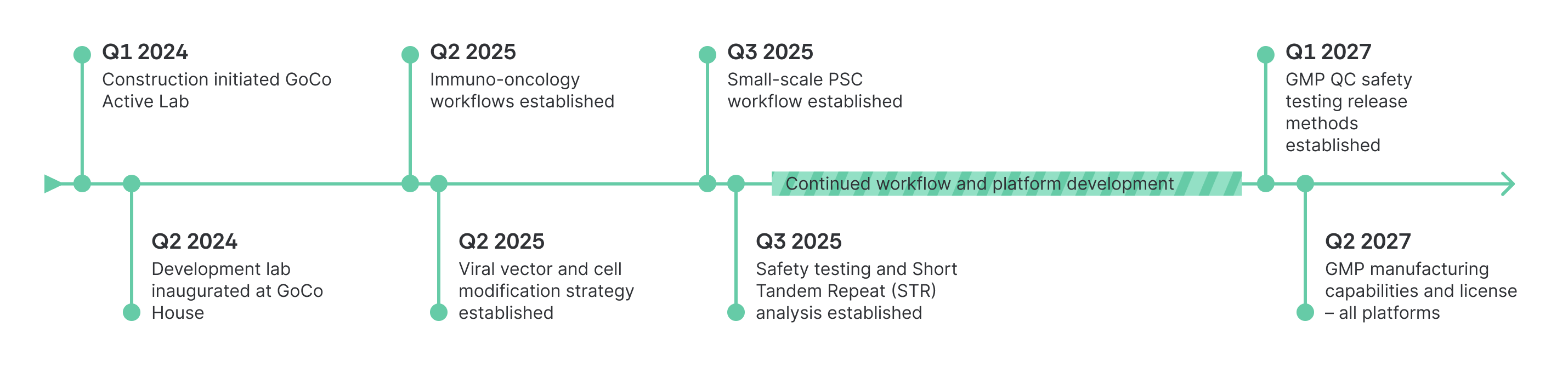
Development Milestones and Roadmap
Our GMP facility is under construction and progressing on schedule. This roadmap highlights the key milestones on our path to becoming fully operational in Q2 2027.
Stay Connected with CCRM Nordic
Join our network for exclusive insights, career opportunities, and thought leadership content from Nordic industry experts.
- 4000+ professionals
- Direct communication
- Weekly insights
Discover Our Other Areas of Support
-

Analytical Services
Safety, identity, and molecular testing to support reliable ATMP development.
Read more -

Market and IP Services
Market and IP services to secure strategy and prepare ATMPs for commercialization.
Read more -

Company Creation and Financing
Supporting ATMP innovation by developing ventures and securing vital funding.
Read more
